
An apparatus that permits observation of the specimen and stirring element during the test is preferable. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smoothly rotating stirring element. The water bath or heating device permits holding the temperature inside the vessel at 37 ± 0.5 during the test and keeping the bath fluid in constant, smooth motion. The vessel is partially immersed in a suitable water bath of any convenient size or heated by a suitable device such as a heating jacket. The assembly consists of the following: a vessel, which may be covered, made of glass or other inert, transparent material 1 a motor a metallic drive shaft and a cylindrical basket. For media with a pH of 6.8 or greater, pancreatin can be added to produce not more than 1750 USP Units of protease activity per 1000 mL. Where water or a medium with a pH of less than 6.8 is specified as the Medium in the individual monograph, the same Medium specified may be used with the addition of purified pepsin that results in an activity of 750,000 Units or less per 1000 mL. For hard or soft gelatin capsules and gelatin-coated tablets that do not conform to the Dissolution specification, repeat the test as follows. Where the label states that an article is enteric-coated, and where a dissolution or disintegration test that does not specifically state that it is to be applied to delayed-release articles is included in the individual monograph, the procedure and interpretation given for Delayed-Release Dosage Forms is applied unless otherwise specified in the individual monograph.
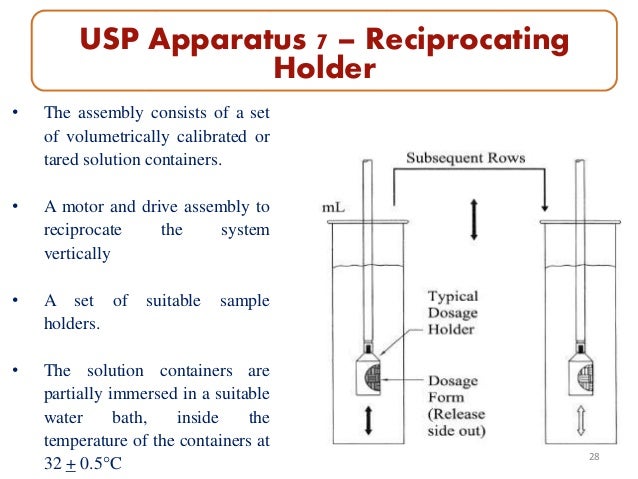
Of the types of apparatus described herein, use the one specified in the individual monograph. In this general chapter, a dosage unit is defined as 1 tablet or 1 capsule or the amount specified. This test is provided to determine compliance with the dissolution requirements where stated in the individual monograph for dosage forms administered orally.


 0 kommentar(er)
0 kommentar(er)
